Basic information and some background on the atom and elements are essential in understanding this lesson. Address any student deficiencies and review them appropriately before teaching the lesson. Students must know the following (see S-C-5-1_Background Notes in the Resources folder):
- Matter is anything that takes up space and has mass.
- Elements are substances that cannot be separated into simpler substances. Each element consists of only one type of atom.
- Atoms are the smallest units of an element that have all of the properties of that element.
- All atoms are made up of small particles called subatomic particles, including:
o Electrons have a negative charge.
o Protons have a positive charge.
o Neutrons have a neutral or no charge.
- Protons and neutrons are packed together in the nucleus, which has a positive charge.
- Electrons are found in a region surrounding the nucleus called the electron cloud, which has a negative charge.
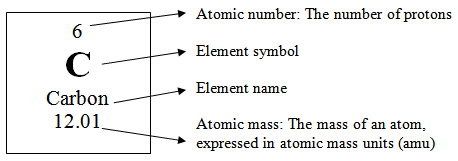
- Atomic Number is the number of protons in an atom.
- Mass Number is the sum of protons and neutrons in an atom.
- Isotopes are atoms of the same element with the same atomic number, but different atomic masses, so they have a different number of neutrons.
To prepare for this lesson, print out three sets of Element Cards: Parts 1–3 (S-C-5-1_Element Cards.pdf). Take down any periodic tables posted in the classroom.
Optional: Read the following passage as an introduction to the lesson:
“Through thousands of years, people have searched for the fundamental building blocks of matter. Empedocles, a scientist who lived in Sicily between 492 BCE and 432 BCE, proposed a theory that attempted to describe the things around us. Empedocles argued that all matter was composed of four elements: fire, air, water, and earth. The ratio of these four elements affected the properties of the matter.
A few decades later, a Greek philosopher named Democritus, developed a theory that all matter can be broken down into tiny indivisible units called atomos. He formed this theory by repeatedly cutting a stone into smaller and smaller halves. This theory, though correct and accepted today, took hundreds of years to gain acceptance because Aristotle believed Empedocles’ theory of the four key elements. It wasn’t until the 17th and 18th centuries that the theory that matter consisted of small indivisible particles was accepted as a result of scientific breakthroughs.
As more and more elements were discovered, so were their properties. Scientists noticed that many elements shared the same or similar properties and grouping them into specific categories became a difficult task. In the late 19th century, Julius Lothar Meyer, a German scientist, and Dmitri Mendeleev, a Russian scientist, developed a way to categorize the known elements. The influential contribution made by Mendeleev was the table’s ability to predict elements that had not been discovered yet.”
Organize students into groups of three or four, and hand out or show copies of both Mendeleev’s and Meyer’s original tables to the class. Ask the class to find similarities and differences between the tables (S-C-5-1_Mendeleev’s Periodic Table.doc and S-C-5-1_Meyer’s Periodic Table.doc). Collect student responses and discuss possible differences between the two tables. (Responses will vary but touch on the fact that Meyer’s table only listed 28 elements and that he classified his table based on valence, the atom’s ability to form charges, rather than atomic mass like Mendeleev’s table. Mendeleev also left gaps where he predicted the existence of undiscovered elements.) Have students put their copies of the tables away before beginning the next activity.
Part 1
Hand out copies of the Pre-lab Questions to each student (S-C-5-1_Pre-lab Questions and KEY.doc). Allow several minutes for students to answer the pre-lab questions and then discuss the answers with the class.
Hand out copies of Element Cards, Part 1 and graph paper to each group (S-C-5-1_Element Cards.pdf and S-C-5-1_Graph Paper.pdf) and copies of the Post-lab Questions to each student (S-C-5-1_Post-lab Questions and KEY.doc).
Tell students, “Each group has a portion of the known elements at the time that Mendeleev and Meyer were working on organizing the elements in 1869. Read through each card carefully and arrange the cards into a table, based on the properties listed on each card. When your group is finished arranging the Element Cards into a table, make a drawing of your table on graph paper and begin to answer the post-lab questions on the handout. Be sure that your table includes headings or labels to indicate how the elements are arranged.”
When finished, have each group present its table to the class and share answers to the Post-lab Questions (S-C-5-1_Post-lab Questions and KEY.doc).
When all presentations are finished, address the group responses to number 4 and review the answers with the class. Address student questions as necessary:
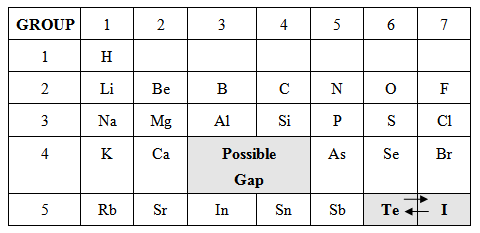
- “Which elements, if any, did not follow these trends? Describe how you arranged these elements.” (A possible answer is that iodine and tellurium break the trend when arranging the elements by ascending atomic masses. Iodine and tellurium switch places when you try to fit them according to other properties. For example, iodine fits better with fluorine, chlorine, and bromine while tellurium fits better with selenium, sulfur, and oxygen.)
Tell the class, “Mendeleev made the same switch with iodine (I) and tellurium (Te). He thought that the atomic masses had been measured incorrectly at the time of his work, and future work would prove that iodine was heavier. Since protons were not discovered yet, he based his organization on atomic mass, instead of atomic number.”
Show the class a modern periodic table or have students take out their copies. Ask them to find iodine and tellurium, and come up with a reason why iodine comes after tellurium even though it has a lower atomic mass. Collect student responses and address questions.
Explain, “The ascending order of the periodic table is based on the number of protons in each element, which is called its atomic number. Its mass number is the sum of its protons and neutrons, and the atomic mass shown is based on the average of all the isotopes of that element, which have different numbers of neutrons. Tellurium has a lower number of protons but heavier isotopes, which increase its atomic mass.”
Have students put away their copies of the periodic table and take down any posted periodic tables before moving on with the lesson.
Ask students, “What gaps or holes did your group find in your tables from the lab activity?” Collect student responses and show an example of a table, highlighting the gaps while reviewing the answer to question 5:
- “Does your table have any holes or gaps? Where are they located on your table? What do these holes or gaps mean?” (Possible gaps may be present in the families or groups of boron and aluminum, and carbon and silicon. The gaps/holes may mean the presence of undiscovered elements at the time of Meyer and Mendeleev.)
Explain to the class that Mendeleev met criticism for predicting the existence of undiscovered elements. Some scientists didn’t value the structure in which he organized the elements, which later proved to be correct in predicting the existence of elements. In 1875, a French scientist named Paul-Emile Lecoq de Boisbaudran discovered gallium and later in 1886, a German scientist named Clemens Winkler discovered germanium.
Part 2
Tell students, “This part of the lesson is designed to see if Mendeleev’s theory of the arrangement of elements was strong enough to be able to predict the existence of unknown elements by holding a place within his periodic table for them.”
Hand out Element Cards, Part 2 (S-C-5-1_Element Cards.pdf), and have groups place them into their tables and add them to their tables on graph paper along with the other elements from Part 1.
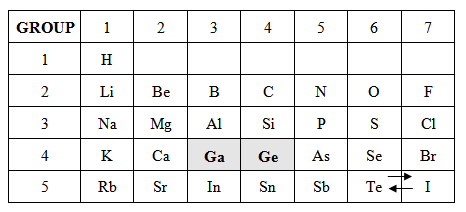
Once students have finished Element Cards, Part 2, address student questions and review the placements of gallium and germanium in their tables. “You should find that gallium fits well into the group with boron and aluminum while germanium fits well with carbon and silicon. What do you think the discovery of these elements and their placement within Mendeleev’s periodic table did for his theory?” Collect student responses before moving to Part 3.
Ask, “What role did the skepticism of critics provide in the overall scientific process of the development of the periodic table?” (Skepticism provides a crucial role in the scientific process. Theories become stronger when they are put through an extensive process of scrutiny.)
Part 3
Tell students, “In the 1890s, Scottish chemist Sir William Ramsay isolated five new gases with the help of other scientists. Place these elements into your tables.” Hand out Element Cards, Part 3, and have students modify their existing tables with the new cards.
When the groups are done, ask students the following questions and collect student responses:
- “Did these new elements fit into your tables?”
- “If not, where did you place them and what do they all have in common?”
(Answers may vary. Students should mention that these new elements belonged in a new group on their tables and that they represent gases.)
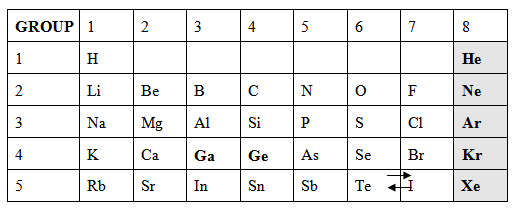
When all groups are finished organizing their tables, hand out the Extension Questions (S-C-5-1_Extension Questions and KEY.doc). Allow students to compare their tables with a modern periodic table. Direct them to find similarities and difference between the tables.
Allow students time to finish the questions. The Extension Questions may be given for homework or at the end of the class. Collect the Pre-lab, Post-lab, and Extension Questions for individual assessment.
Extension:
- Students who might need an opportunity for additional learning can use a copy of the Background Notes (S-C-5-1_Background Notes.doc).
- Students who may be going beyond the standards can create a timeline table, consisting of the following:
o history of the atomic theory.
o models of the atom.
o history of the discovery of known elements (element name, discoverer, and year).
o timeline organized according to visual depictions in a PowerPoint presentation, story, or drawing.
