Day 1: Solubility of Solids Lab
Prior to the lesson, set up the materials for the Solubility of Solids Lab (S-C-9-2_Solubility of Solids Lab and KEY.docx).
Introduce the lab by reviewing the previous lesson on solubility and the relationship it has with temperature.
Ask the following questions, taking time to review the correct answers and address any additional student questions:
- “What are the components of a solution?” (a solute and a solvent)
- “What are the three types of solutions? Describe each.” (Saturated: a solution that contains the maximum quantity of solute that dissolves at that temperature. Unsaturated: a solution that contains less than the maximum amount of solute that can dissolve at a particular temperature. Supersaturated: a solution that contains more solute than a saturated solution.)
- “What is the relationship between temperature and solubility of a solid?” (An increase in temperature increases the amount of solvent that will dissolve into a solvent.)
- “How do you create a supersaturated solution from a solution already at its saturation point?” (increase the temperature)
Hand out to students copies of the lab instruction sheet (S-C-9-2_Solubility of Solids Lab and KEY.docx) and graph paper (S-C-9-2_Graph Paper.pdf). Instruct students to take out two colored pencils.
Assign groups of 2–4 students, depending on lab space and resources. Read through the materials and procedure sections as a class. Review safety guidelines and have all students put on their goggles and gloves. Address student questions before starting the lab. Monitor student work and participation throughout the lab. Collect lab reports when students finish.
Day 2: Solubility of Gases Lab
Prior to the lesson, set up the materials for the Solubility of Gases Lab (S-C-9-2_Solubility of Gases Lab and KEY.doc).
Say, “In our first lesson on solubility, we briefly learned that the solubility of gases acts differently than solids. The solubility of solids is dependent on temperature. The higher the temperature, the more soluble the solution is. The solubility of gases depends on temperature as well, but the difference is that it decreases as temperature increases. The solubility of gases is also dependent on pressure. It increases as pressure increases.” Display the diagrams below for students:
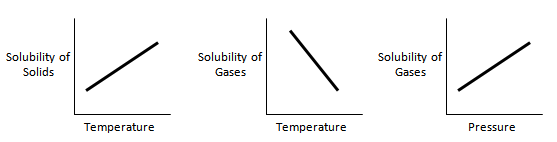
“Do you notice how the solubility of solids and gases act differently as temperature increases? Do you notice how an increase in pressure increases the solubility of gases?”
Show the class the Solubility of Gases Presentation (S-C-9-2_Solubility of Gases Presentation PowerPoint.pptx). Either project the presentation with a document camera or LCD projector, or print copies of the PDF version for each student (S-C-9-2_Solubility of Gases Presentation PDF.pdf).
Instruct students to take notes throughout the presentation and write down any questions they have. When finished with the presentation, address student questions before moving on.
Ask, “Why does the solubility of gases decrease as temperature rises and increase with more pressure?” Collect student responses and have them copy the following information into their notes:
Solubility vs. Pressure vs. Temperature
- Increased temperature causes an increase in kinetic energy, which is the movement of the molecules in the solution. The higher kinetic energy causes more motion in molecules, which run into one another and break intermolecular bonds, allowing gases to escape from the solution.
- Lower temperatures cause a lower kinetic energy, which causes intermolecular bonds to strengthen, which holds on to more gases.
- Increasing the pressure causes more force to be applied to the solution, keeping the gases in the solution.
- Decreasing the pressure causes less force to be applied to the solution, weakening its “hold” on the gases, allowing them to escape. Opening a can of soda quickly reduces the pressure, and the bubbles floating to the surface are gas molecules escaping into the atmosphere.
Hand out copies of the Solubility of Gases Lab (S-C-9-2_Solubility of Gases Lab and KEY.doc).
Assign groups of 2–4, depending on lab space and resources. Read through the materials and procedure sections as a class. Review safety guidelines and have all students put on their goggles. Address student questions before starting the lab. Monitor student work and participation throughout the lab. Collect lab reports when students finish.
Extension:
- For students performing beyond the standards, have them research the Gas Laws that cover the relationship between the solubility of gases in a solution under varying environmental changes. Students create a presentation (PowerPoint, poster, oral, etc.) explaining the gas laws as well as the solubility of gases under these varying environmental changes.
o Boyle’s Law
o Charles’s Law
o Combined Gas Law
o Ideal Gas Law
- For students requiring more practice with the standards, provide them with a copy of the presentation (S-C-9-2_Solubility of Gases Presentation PDF.pdf). Students may use this handout as a reference throughout the lesson. Students may also present their answers visually by drawing a picture representing the solubility of gases instead of written responses.
