Introduce the lesson by displaying a large poster of a periodic table or handing out individual copies to students (S-C-5_Modern Periodic Table.pdf). Ask the class the following questions and collect student responses to warm up. Ask,
- “What do you think early versions of this table looked like?”
Answers may vary quite a bit. Remind students of Mendeleev’s table and how it may have changed. Possible responses include less known elements, and possible holes in the table for the future discoveries based on different properties.
- “What are reasons for the layout of the periodic table?”
Answers may vary. Students should mention that the layout can be based on atomic number, atomic mass, similar properties in vertical rows, electron configurations, ability to bond with other elements, etc.
- “What are some differences between hydrogen (H) and lead (Pb)?”
Answers may vary. Students should mention differences in atomic mass, atomic number, physical and chemical properties, electron configuration, ability to bond with other atoms, etc.
Say, “You will be introduced to the layout and organization of the modern periodic table. It may look confusing at first, but this ingenious table serves as the most important resource in all of chemistry.”
Briefly review the following information and address student questions from Lesson 1:
- Periodic table was first developed in 1800s.
- Dimitri Mendeleev published one of the first tables in 1869, which led scientists to today’s arrangement.
- First tables were organized by increasing atomic mass.
- Now, the periodic table is organized by the increasing atomic number.
- Early versions helped scientists discover new elements.
Hand out copies of a blank periodic table (S-C-5_Blank Periodic Table.pdf) and instruct students to take out 8 colored pencils and their notebooks for this lesson. Colors are arbitrary, but they need 8 different colors. Note: Colored pencils and highlighters work best because the element descriptions can be seen over the color.
Say “I am going to review the layout and organization of the periodic table and you are going to color code your blank tables as we go along.”
Project the following information for the class, or write it on the board and instruct students to copy down the information into their notebooks:
“The Layout of the Periodic Table
The table is split into horizontal rows and vertical groups:
- Group: A column in the periodic table; elements in one group have the same number of electrons in the outermost energy level. Elements in a group have similar chemical properties.
- Period: A row in the periodic table.”
Part 1: Groups
Say, “I will project different sections of the periodic table. Your job is to color in the vertical columns (groups) with different colors of your choice and copy down the element symbols into the appropriate box. You will also copy down all information on each group in your notebooks. From now on, we will be referring to vertical columns as groups and horizontal rows as periods.”
A Periodic Table Groups handout is found in the Resources folder (S-C-5-2_Periodic Table Groups.doc). Project these documents on an overhead or LCD projector or hand out individual copies to students.
Part 2: Periods in the Periodic Table
Say, “Now that we have our groups with similar chemical properties established, it’s time to organize the Periods (horizontal rows). As you move from left to right along the periodic table, the atomic number and atomic mass increase. The same applies as you move down the groups. The increase in atomic mass is due to an increase in protons, which is balanced by a proportional increase in the number of electrons.”
Hand out copies of the Modern Periodic Table (S-C-5_Modern Periodic Table.pdf), or project a copy to the whole class. Have students use the periodic table to fill in their own. Instruct students to do the following:
- Label each group 1–18.
- Number each element with the atomic number on top of the symbol.
- Write the full element name under the symbol.
- Number each element with the atomic mass below the element name.
Each box should look like the following:
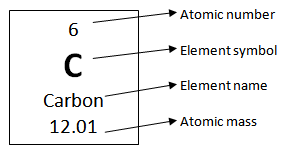
Address any student questions throughout this activity and monitor students to make sure they are filling in their tables correctly. Once finished, say “As a reminder, the atomic number stands for the number of protons in the atom. The number of electrons is equal to the number of protons and the number of electrons is the basis for why each element is placed horizontally on the periodic table. We call this arrangement the electron configuration.” The electron configuration describes the arrangement of electrons within an atom and the location of electrons within different orbitals surrounding the atom.
Have students copy the following in their notebooks:
“There are 7 periods in the periodic table. Each period stands for a new energy level in the electron configuration, which is an area around the nucleus of an atom where a specific number of electrons exist. There are rules about how many electrons can fit into each shell, and they are described in the table below (1s, 2s, 3s, 4s, etc.). Each energy level, besides the first energy level, has sublevels. The first period has 1 shell, the second period has 2, the third period has 3, and so on.”
|
Energy Level or Sublevel
|
1s
|
2s
|
2p
|
3s
|
3p
|
4s
|
3d
|
|
Maximum # of Electrons
|
2
|
2
|
6
|
2
|
6
|
2
|
10
|
|
What Does It Mean?
|
2
electrons can fit here
|
2 electrons can fit here
|
6 electrons can fit here
|
2 electrons can fit here
|
6 electrons can fit here
|
2 electrons can fit here
|
10 electrons can fit here
|
For example,
- “Hydrogen (H) has 1 electron, so its electron will go into the first energy level, and into the s sublevel (i.e., 1s sublevel). It has an electron configuration of 1s1.
- Helium (He) has 2 electrons, so its electrons will fill up the first energy level. Its configuration is 1s2.
- Lithium (Li) has 3 electrons, so two of its electrons will fill up the first energy level and one electron will be in the second energy level. Its configuration is 1s22s1.
- Beryllium (Be) has 4 electrons, so two of its electrons will fill up the first energy level and the other two electrons will fill the second energy level. Its configuration is 1s22s2.
- Boron (B) has 5 electrons, so two of its electrons will fill up the first energy level, two electrons will fill the second energy level, and one electron will be in the next energy level. Its configuration is 1s22s22p1.
- Carbon (C) has 6 electrons, so its electrons will fill up the 2 electrons in the first energy level (1s), 2 electrons in the second energy level (2s), and 2 out of the 6 electrons in the 2p sublevel. Its configuration is 1s22s2p2.
- Potassium (K) has 19 protons and electrons, so its configuration is 1s2 2s2 2p6 3s2 3p6 4s1. Notice how each energy level and sublevel were filled in order?”
For a complete list of electron configurations, refer to the Electron Configurations document (S-C-5_Electron Configurations.pdf).
Have students copy the table below:
|
Energy Level/Sublevel
|
Sublevel
1s
|
Sublevel
2s
|
Sublevel
2p
|
Sublevel
3s
|
Sublevel
3p
|
Sublevel
4s
|
Sublevel
3d
|
|
Maximum # of Electrons
|
2
|
2
|
6
|
2
|
6
|
2
|
10
|
|
H
1 electron
|
1s1
|
|
|
|
|
|
|
|
He
2 electrons
|
1s2
|
|
|
|
|
|
|
|
Li
3 electrons
|
|
1s22s1
|
|
|
|
|
|
|
Be
4 electrons
|
|
1s22s2
|
|
|
|
|
|
|
B
5 electrons
|
|
|
1s22s2p1
|
|
|
|
|
|
C
6 electrons
|
|
|
1s22s2p2
|
|
|
|
|
|
N
7 electrons
|
|
|
1s22s2p3
|
|
|
|
|
|
O
8 electrons
|
|
|
1s22s2p4
|
|
|
|
|
|
F
9 electrons
|
|
|
1s22s2p5
|
|
|
|
|
|
Ne
10 electrons
|
|
|
1s22s2p6
|
|
|
|
|
|
K
19 electrons
|
1s2
|
2s2
|
2p6
|
3s2
|
3p6
|
4s1
|
|
|
Cu
29 electrons
|
1s2
|
2s2
|
2p6
|
3s2
|
3p6
|
4s1
|
3d10
|
Ask the class, “How can you tell that helium has a filled outermost energy level?” Collect responses and review with students that helium has 2 electrons in the outermost energy level, which is its only energy level, and the energy level only has room for 2 electrons, so it is filled. Explain, “The electrons in the outermost energy level are called valence electrons. These valence electrons are the basis for the groups that each element belongs to in the periodic table. Every element in a certain group has the same number of valence electrons in its outer most energy levels.”
Ask, “Can anyone tell me another element that has the same number of valence electrons as hydrogen?” Again, collect responses before reviewing that all elements in Group 1A have 1 valence electron (i.e., H, Li, Na, K, Rb, Cs, and Fr).
“Elements in Group 18 are referred to as the noble gases. Helium is a member of these gases. They are unique in that their outer energy levels are filled with electrons. This makes them very non-reactive compared to other elements.
Elements in Group 17 are missing one electron each in order to be filled, which conversely, makes them highly reactive compared to other elements.”
Below are some examples of other electron configurations. For a complete list, refer to the Electron Configurations document (S-C-5_Electron Configurations.pdf).
|
Element
|
Number of Electrons in the Element
|
Electron Configuration
|
|
He
|
2
|
1s2
|
|
Li
|
3
|
1s22s1
|
|
Be
|
4
|
1s22s2
|
|
O
|
8
|
1s22s22p4
|
|
Cl
|
17
|
1s22s22p63s23p5
|
|
K
|
19
|
1s22s22p63s23p64s1
|
Provide time for students to ask questions and review electron configurations of more elements of their choice. When finished, have students copy the following information:
“Valence electrons:
- are the electrons in the outermost energy level.
- are involved in bonding.
- are the electrons that are lost/added when an atom forms an ion.
- atoms will lose or gain electrons in order to have a full sublevel… like the noble gases. Valence electrons for H, He, Li, C, N, and Na:”
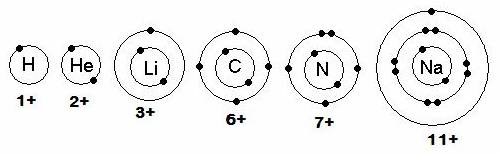
Source: http://www.mansfieldct.org/schools/mms/staff/hand/AtomicStructure_files/image002.jpg
Hand out copies of the Element Organization Activity and Cards (S-C-5-2_Element Organization Activity and Rubric.doc and S-C-5-2_Element Organization Activity Cards.doc). Say, “This activity will help you get oriented with the layout of the periodic table the characteristics of the first 36 elements.”
Allow students time to work on this activity in class or as homework. Collect poster boards for individual assessment.
Extension:
- Students who might need an opportunity for additional learning can use a filled-out periodic table, consisting of groups, periods, elemental characteristics, electron configuration, etc., to use a reference throughout the lesson.
- Students who may be going beyond the standards can research the electron configurations of metals, lanthanide series, and actinide series to see why they are different than other groups.
