
Safety Precautions: Students should NOT be allowed to handle concentrated sulfuric acid. It is extremely irritating to skin and eyes, and highly reactive. You will dilute 18M sulfuric acid to 3M sulfuric acid. Add the acid VERY slowly to cold water with ice cubes in it, stirring constantly. If the heat builds up, it can spray onto skin and cause severe burns.
Advance Preparation: Prepare diluted sulfuric acid solution. Practice the “Elephant Toothpaste” demonstration before class.
Tell students that you are going to perform a demonstration for them. The demonstration represents a chemical reaction. They will see evidence that a chemical reaction is taking place (color change, formation of a precipitate, formation of gas, production of heat, etc.). As you work through the demonstration, write the products and reactants on the board. Students should copy this information as well as their observations into their notebook.
Demonstration: “Elephant Toothpaste”
Using a 500 ml glass graduated cylinder (smaller will work, but will not be as theatrical), add 5 ml of liquid dish soap and 20 ml of concentrated (30%) H2O2. Stir. If you do not have access to concentrated hydrogen peroxide, you can use the H2O2 from a local store, keeping in mind it is only 3%. To make the end product look like toothpaste, you can slide two drops of red food coloring down one side of the cylinder. Then slide two drops of green food coloring down the other side of the cylinder. When you are ready, add 5 ml of 2M potassium iodide (KI).
As you are adding the reactants, ask students to write them down so that they have practice differentiating between reactants and products. Have students write down their observations. They should notice that the reaction produces a considerable amount of heat and bubbles. You may need to help them write the products of this reaction as shown below. Do not balance the equation. Based on Lesson 1, students should be able to create an atom inventory and balance the reaction; guide them through this if needed.
Unbalanced Reaction: H2O2 KI O2 + H2O
-
Balanced Reaction: 2H2O2KI O2 + 2H2O
Ask students, “What observations did you make?” Field several answers and explain why each observation occurred. Most students will notice the bubbles or foam produced. The oxygen gas gets trapped in the soap, which produces the foam. In fact, if you light a wooden splint (coffee stirrer) with a match, blow it out so that it glows and gently hold it over the bubbles, it will relight. Some students may notice the foam steaming. If they are close to the demonstration, they may notice it feels hot. This is a result of the reaction being exothermic. This would be a good time to define exothermic reaction:
-
Exothermic reactions: Chemical reactions that produce heat or light as the reaction proceeds. They often feel warm to the touch. Exothermic reactions are often written with energy as one of the products, such as:
-
2H2O2 KI O2 + 2 H2O + Energy
-
Tell students, “Chemical reactions, such as the one in the elephant toothpaste demonstration, can be classified into categories. There are several categories that chemists typically refer to but today we are going to focus our discussion on five of them. Please write the names in your notebook. Leave enough space to write the definition and examples for each type of reaction.” Write on the board:
Decomposition
Synthesis
Single Replacement
Double Replacement
Combustion
Decomposition Reactions
Say, “Let’s examine the elephant toothpaste reaction. How many reactants does the reaction have? How many products are there?” Students should respond with one and two, respectively. Say, “This reaction is classified as a decomposition reaction, because one reactant, in this case H2O2, is breaking down into two or more simpler products (i.e., O2 and H2O). Please write this definition in your notebooks. Before we go through specific examples, here is a generic formula you can apply to decomposition reactions.” Write on the board:
AB A + B
Note: AB represents a compound; A and B represent simpler compounds or elements.
“Here is a specific example” (write on the board):
2Cl2O5 2Cl2 + 5O2
**Some students may ask if there is one reactant in this example or two, due to the coefficient of 2 in front of dichlorine pentaoxide. Explain that there is still one type of reactant, but two sets of it.
6NaHCO3 3Na2CO3 + 3H2O + 3CO2
Synthesis Reactions
Ask students, “Looking at the list of reaction types, which one do you think is the opposite of decomposition?” (Synthesis is the correct answer. Synthesis reactions are sometimes called “composition reactions.”) “The word synthesis means to make something new. Instead of starting with one reactant, one product will be formed from two or more reactants. Write that definition down in your notebooks. Using the generic formula we used for a decomposition reaction can you generate a generic formula for a synthesis reaction?” Write on the board:
A + B AB
“Here are some specific examples” (write on the board):
O2 + 2H2 2H2O
2Na + Cl2 2NaCl
Single- and Double-Replacement Reactions
Say, “As you can see, synthesis and decomposition reactions are related. Single-replacement and double-replacement reactions are related as well. In a single-replacement reaction, an uncombined element will replace a less reactive element in a compound, creating a new compound and a single element. The generic formula for this reaction type is as follows” (write on the board):
AB + C B + AC (if C forms a negative ion)
AB + C A + CB (if C forms a positive ion)
Metal and nonmetal single replacements examples:
-
Metal + oxygen → metal oxide
-
EX. 2Mg(s) + O2(g) → 2MgO(s)
-
Nonmetal + oxygen → nonmetallic oxide
-
EX. C(s) + O2(g) → CO2(g)
-
Metal oxide + water → metallic hydroxide
-
EX. MgO(s) + H2O(l) → Mg(OH)2(s)
-
Nonmetallic oxide + water → acid
-
EX. CO2(g) + H2O(l) → ; H2CO3(aq)
-
Metal + nonmetal → salt
-
EX. 2 Na(s) + Cl2(g) → 2NaCl(s)
Source: http://www.files.chem.vt.edu/RVGS/ACT/notes/Types_of_Equations.html
“Recall that chemists refer to negative ions as anions and positive ions as cations. Let’s label each element as either a cation or anion.” Write the equation on the board:
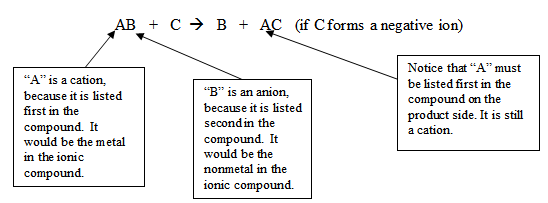
Continue by saying, “It may help to think of single-replacement reactions as a couple dancing (AB) and a single person cutting in (C), making a new pair (AC) and leaving someone out (B).” You can use a famous couple, such as Jennifer Aniston and Brad Pitt as the couple (AB) and Angelina Jolie as the unpaired element (C) to add a dose of humor to the lesson.
Add, “The definition I gave you stated that an uncombined element will replace a less reactive element in a compound, creating a new compound and a single element. How do you know which elements are more or less reactive than others? Chemists have created an experimental list of common metals that are placed in specific positions due to their tendency to react. The list is called a metal reactivity series. It places more reactive metals above less reactive metals. Here is an example of a metal reactivity series” (share with students the following chart):
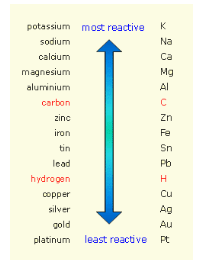
“Notice how potassium (K) is listed above calcium (Ca) on the list. Look at the following single-replacement reaction” (write on the board):
“Notice that potassium, which forms a cation, switches places with calcium, which also forms a cation. Potassium will do this because it is more reactive than calcium (refer to the activity series chart above) and is less stable than calcium when it is alone. It is more stable as a part of a compound, so it will replace calcium (Hence the name, single-replacement reaction). If the reaction were written as…” (write on the board):
“It would not occur, because calcium is not above potassium on the reactivity series. Let try another example.” Write the equation on the board:
“Ask yourselves, will this reaction happen? Refer to the activity list. Aluminum is the uncombined metal (cation) here. Is it above the metal in the compound (copper in this case)? The answer is yes, so the reaction happens as written. Aluminum, the cation, is replacing copper, the other cation, in a single-replacement reaction.”
Continue with double-replacement reactions by saying, “Double-replacement reactions are similar to single-replacement reactions in that you need to be conscientious of cations and anions. However, you will not need to use the metal reactivity series. Here is an example of a generic formula for double-replacement reactions” (write on the board):
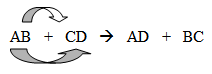
“What are the two cations? What are the two anions? How do you know?” (The cations are A and C because they are written first in the ionic pair. B and D are the anions because they are written second in the ionic pair.) Continue by saying, “Notice how the cations are switching places and the anions are as well. You start with two compounds and finish with two new compounds. Here are more examples.” Write the following equations on the board:
3MgCl2 + 2AlN Mg3N2 + 2AlCl3
CaO + 2KI CaI2 + K2O
2NaF + ZnS Na2S + ZnF2
Combustion Reactions
Finally, say, “Combustion reactions are different from the other reactions we have covered in that they usually follow a very specific formula. The reactants are a hydrocarbon (in this lesson) and oxygen and the products are carbon dioxide and water. The backbone is as follows” (write on the board):

Continue by saying, “Often, chemists will refer to a molecule burning in oxygen when describing a combustion reaction. They are usually exothermic reactions. What does that mean?” (They produce heat.) “Here are some examples.” Write the equations on the board.
C10H8 + 12O2 10CO2 + 4H2O + E
C6H12O6 + 6O2 6CO2 + 6O2 + Energy (cellular respiration)
Laboratory: Reaction Types
Guide students in the five types of reactions lab. Hand out copies of the Reaction Types Lab Worksheet (S-C-3-2_Reaction Types Lab and KEY.doc) and the Data Table (S-C-3-2_Reaction Types Lab Data Table.doc). Some of the information gathered during this lab will be utilized during Lesson 3.
Extension:
-
During the “Elephant Toothpaste” demonstration, potassium iodide is used as a catalyst. You may choose to discuss the role of catalysts in chemical reactions and relate catalysts to enzymes used in biochemical reactions.
-
During the “Elephant Toothpaste” demonstration, energy was produced. You may wish to incorporate a discussion of reaction thermodynamics such as enthalpy, entropy, and heat of combustion (See Related Resources.)
-
Students who may be going beyond the standards during the lab can capture the hydrogen gas from the reaction and perform a hydrogen “pop test” (See Hydrogen Pops in Related Resources for a detailed procedure and explanation.)
-
You may wish to discuss more chemical reaction types such as acid-base and nuclear reactions.
-
It may be helpful to include charges in reactions that involve cations and anions.
-
You may wish to discuss how chemists note the state of matter for each reactant and product such as:
-
(g) gas
-
(s) solid
-
(aq) aqueous
-
(l) liquid
