Much of this lesson requires students to use
previous knowledge involving writing ionic formulas. They may need to
start with some review, which is shown below.
Ionic Compounds Nomenclature
-
Metals (cations) are listed first,
followed by nonmetals (anions).
-
When writing ionic formulas, the final
compound needs to be neutral.
-
Reference the Periodic Table to
determine the ion that each atom forms.
- The name of the metal stays the same, but
the ending on the anion changes to
- “ide.”
-
For transition metals that can have more than
one charge, the charge on the metal is indicated with Roman
numerals.
-
For compounds with polyatomic ions, the
polyatomic ion names stay the same. The endings are not changed to
“ide.”
Writing Formulas
Show students how compounds are formed,
using the crisscross method with the ions.
-
A) Li and S Li+1 and
S-2 Li2S
There must be two lithium ions for every one
sulfur ion to make a neutral compound.
B) Zn and N Zn+2 and N-3 Zn3N2
There must be three zinc ions for every two
nitrogen ions to make a neutral compound.
C) Al and OH Al+3 and
OH-1 Al(OH)3
There must be three hydroxide ions for every
one aluminum ion to make a neutral compound.
Naming Examples
A) NaCl sodium chloride
B) Mg3N2 magnesium
nitride
C) CaI2 calcium iodide
D) KCl potassium chloride
E) NH4OH ammonium hydroxide
F) Na2SO4 sodium
sulfate
G) LiOH lithium hydroxide
If you feel that some students need
reinforcement, have them complete the Ionic Compounds Practice
worksheet (S-C-3-3_ Ionic Compounds Practice Worksheet and KEY.doc).
Once you have reviewed writing and naming
ionic compounds, you are ready to begin the lesson on predicting
products. This lesson works through each type of reaction as a means
to understanding the products.
Say, “During this unit, you have
learned to balance chemical equations and classify chemical
reactions. Now, your task is to predict what the products of those
chemical reactions will be and write balanced equations. We will work
through all five reaction types and predict what the products will
be. When we are done, your lab group from yesterday will meet to
analyze the products from the lab’s reactions. Let’s start by
looking at a decomposition reaction first.” Write on the board:
CO2
Ask students why this reaction would be
classified as a decomposition reaction. They should recognize that it
only has one reactant. Ask, “What are the elements from which
CO2 is composed? Once you determine the
atoms that make up CO2, you will write them
separately as products, like this” (write on the board):
CO2
C + O
Continue with, “Some of you may notice
that the reaction does not satisfy the law of conservation of matter.
It is not balanced. Before you put a coefficient of 2 in front of the
oxygen on the product side, you must know whether carbon or oxygen is
a diatomic element. Please record the definition of this in your
notes.” Provide students with the following definition:
Diatomic elements: Chemical elements
whose stable form at STP consists of diatomic molecules. The
diatomic elements are H2,
N2,
O2,
F2,
Cl2,
Br2,
and I2.
“Notice that oxygen is alone on the
product side but is a diatomic element. This means that oxygen is
unstable alone and will be found in nature as a pair. Therefore, you
will need to add a subscript of 2, not a coefficient of 2 to oxygen.
The only time that you can add a subscript in an equation is when
there is a diatomic element. The equation is now balanced without
adding any coefficients.” Write on the board:
CO2
C + O2
Try these decomposition examples with the
class:
A) H2O2
H2O2
H2 + O2
B) Cu2O
2Cu2O
4Cu + O2
Tell the class, “Now that you can
predict the products of a decomposition reaction, you will be able to
predict the products of a synthesis reaction. Keep in mind that
decomposition reactions are essentially the opposite of synthesis
reactions. Decomposition reactions will have one reactant, whereas
synthesis reactions will have one product. Let’s look at a few
examples.” Write on the board:
A) O2 + H2
H2O2
B) 2Na + Cl2
2NaCl
C) 8Fe + S8
8FeS
Guide students to the next reaction by
saying, “The next two types of reactions are single replacement
and double replacement. They are closely related in terms of how you
will predict the products of the reaction. Let’s try a single
replacement reaction first.” Write on the board:
NaCl + K
Distribute copies of the metal reactivity
series shown below (S-C-3-3_Metal Reactivity Series.doc).They will use it to determine how the reaction will
progress.
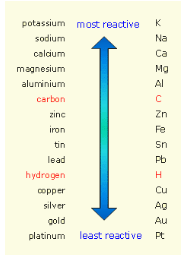
Say, “In the example shown above,
potassium is more reactive than sodium so it will replace sodium and
the reaction will proceed. Potassium forms a +1 cation and
chlorine forms a -1 anion. When they form an ionic compound, it will
be written as KCl. There is a redistribution of charge when metals
and nonmetals are charged (e-). “When you
are predicting the products of a displacement reaction it is
helpful to write out each ion and the charge that it forms. Knowing
that every ionic compound is made from a cation and an anion (metal
and nonmetal), you will form new products with the ions you have.”
Sodium is the element that was replaced and will be written by
itself as one of the products. It is not a diatomic element. The
balanced reaction looks like this. The charges are shown so that you
can see how they are redistributed.” Write on the board:
Na+1Cl-1
+ K0 K+1Cl-1
+ Na0
Ask students to try these practice problems.
Remind them to look for diatomic elements.
A) BaCl2 + 2K
2KCl + Ba
B) 2AlN + 3Cl2
2AlCl3 + N2
Students will now try predicting the
products of a double displacement reaction. Write the following
example on the board:
“It is helpful to draw arrows to show
how the compounds 'switch partners.' Break both ionic compounds apart
into ions.” Begin writing the following equations on the board,
while continuing to instruct students. “Make a list like the one
I’m writing. When you write the new products, write the cation
first and the anion second and then neutralize the compound if
needed.”
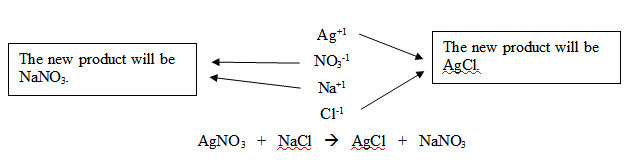
Try a double-replacement reaction using an
ionic compound containing a transition metal like copper. Students
will have to look at the formula given to determine which copper ion
to use.
Cu+2O-2
+ Mg+2Cl2-1
Notice that copper in CuO is the Cu+2
ion. Therefore the balanced reaction will be:
CuO + MgCl2
CuCl2 + MgO
Practice reactions:
A) Li3N + NaCl
-
Li+1
N-3
Na+1
Cl-1
-
Li3N + 3NaCl
3LiCl + Na3N
Introduce the last reaction by saying, “The
last reaction type to predict is a combustion reaction. These are the
most straightforward, because the products will be the same each
time: CO2 and H2O.
You will not incorporate ions because combustion reactions deal with
hydrocarbons, which form covalent bonds. Here is an example.”
Write the following on the board:
CH4 + 2O2
CO2 + 2H2O
Note: For the purpose this lesson, the
products of combustion reactions will ALWAYS be carbon dioxide and
water.
At this time, direct students to form the
lab groups they were in on the previous day. They should work
together to complete the balanced reactions for each of the six
reactions from the lab. Walk around and guide students and answer
questions. Remind them to watch for diatomic elements and to use
their valence sheets (S-C-3-3_Valence Sheet.doc), and also the periodic table to look up the correct charges
for each ion.
Extension:
-
All of the practice reactions require
students to predict products of a chemical reaction using formulas.
You may choose to incorporate the same reactions, including chemical
names as well as formulas. This will allow students to practice
naming formulas.
-
This lesson refers to the metal
reactivity series. You may wish to incorporate a nonmetal reactivity
series with some of the halogens. Information is available at
http://www.nelsonthornes.com/secondary/science/scinet/scinet/reaction/react/halogs.htm
