Part 1
Distribute copies of the Steps for Drawing Lewis Dot Structures handout (S-C-4-1_Steps for Drawing Lewis Dot Structures.docx). Explain that, “A molecule is a neutral group of atoms held together by covalent bonds. Most of the substances we encounter every day, such as water, sugar, and carbon dioxide, are held together by covalent bonds between their atoms. In order to show how covalent bonding occurs we will draw Lewis dot structures. Lewis dot structures are two-dimensional models that we can draw to represent the bonds between atoms in molecules. There are a few rules to keep in mind when drawing these structures.” Explain the steps for creating Lewis dot structures, using the handout and the Periodic table.
Example 1
CH4 (Methane)
|
Atom
|
Valence Electrons
|
|
C
|
4
|
|
H
|
1(4) = 4
|
|
Total electrons
|
8
|
“To make the molecule symmetrical, the carbon atom needs to be in the center, flanked on all sides by hydrogen. Each carbon atom has four valence electrons. Draw each electron as a dot around the carbon atom, like this.” Draw the following on the board:
“Each hydrogen atom has one valence electron. Draw these by the hydrogen atoms.” Draw the following on the board.
http://www.800mainstreet.com/5/0005-002-methane.gif
Explain that, “If you count the number of electrons shown in the valence shell of the carbon atom, there are eight. That is considered a full valence shell. Other atoms that do not have eight valence electrons will form bonds to achieve this full valence shell. This is called the ‘octet rule.’ Hydrogen is an exception to the octet rule, as it needs to have two valence electrons instead of eight to have a full outer shell. When atoms obtain a full shell of valence electrons their stability increases.”
“If you count the total number of electrons in the molecule, there are eight, which matches the total we calculated in the table above. Notice that the hydrogen and carbons atoms are SHARING the electrons in order to achieve a full outer shell of electrons. Let’s try another one.”
Example 2
CCl4 (carbon tetrachloride)
|
Atom
|
Valence Electrons
|
|
C
|
4 (1) = 4
|
|
Cl
|
7(4) = 28
|
|
Total electrons
|
32
|
“Draw the carbon atom with its four valence electrons. Draw the four chlorine atoms around the carbon atom with each of their seven electrons, like this” (draw the following on the board):
http://chemphys.armstrong.edu/P1/a.gif
“Count the total electrons in the molecule, there are 32, which matches the total in our table. Each atom in the molecule also has eight electrons around it. They achieve this stability by sharing their electrons. Meaning, you will count electrons for BOTH carbon and chlorine. The electrons that are being shared can also be written as a single line, or bond. One line represents one single bond. CCl4 can be written like this.” Draw the following on the board:
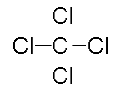
http://chemphys.armstrong.edu/P1/Bonding.html
“In addition to Lewis dot structures, molecules can also be drawn using ‘line-bond structures,’ in which covalent bonds between two electrons are shown as lines joining the two atoms. In this line-bond structure of carbon tetrachloride, the lone pairs of electrons on the four chlorine atoms are not shown but they are understood to be there.”
Example 3
C2H6 (ethane)
|
Atom
|
Valence Electrons
|
|
C
|
4 (2) = 8
|
|
H
|
1(6) = 6
|
|
Total electrons
|
14
|
“Which atom is the central atom in this case? Ask yourself, how can I set the structure up so that is looks the most symmetrical? The two carbon atoms will serve as the backbone. Now draw the valence electrons around each atom.” Begin drawing the model shown below as you guide students.
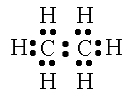
http://www.800mainstreet.com/5/0005-005-mole-formula.htm
“Just as you did in the last examples, count the total number of electrons. It needs to be 14. Look at each carbon atom individually. There needs to be eight electrons around each atom. Look at each hydrogen atom individually. There needs to be two electrons around each atom. Notice how each atom achieves the octet rule, but the total number of electrons stays low. This is only achieved through SHARING the electrons. Replace each electron pair of with a single line to show a bond.”
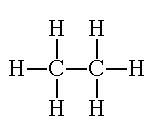
http://www.800mainstreet.com/5/0005-005-mole-formula.htm
Example 4
H2O (water)
|
Atom
|
Valence Electrons
|
|
O
|
6 (1) = 6
|
|
H
|
1(2)= 2
|
|
Total electrons
|
8
|
Instruct students to follow the same steps they have been, in terms of deciding which atom will be the central atom. In this case, the central atom will be oxygen flanked by two hydrogen atoms, because that makes the molecule symmetrical:
http://users.humboldt.edu/rpaselk/ChemSupp/LewisStructures/H2O.gif Instruct students the substitute the electron pairs in between oxygen and hydrogen for single bonds. This can be shown as:
http://upload.wikimedia.org/wikipedia/commons/3/33/Chemical_Principles,_H20_Molecules.png
“Notice that there are two electron pairs around oxygen that are not shared with hydrogen. These are called ‘lone electron pairs.’”
Example 5
CO2 (carbon dioxide)
|
Atom
|
Valence Electrons
|
|
C
|
4 (1) = 4
|
|
O
|
6(2) = 12
|
|
Total electrons
|
16
|
“If each of the three atoms were to have eight electrons, that would be 24 electrons total. You only have 16 electrons to work with, so it is clear that some electrons will have to be shared. However, more electrons will have to be shared between the atoms than were in the previous examples. Start by making carbon the central atom, flanked by two oxygen atoms to make the molecule symmetrical. Then, add the valence electrons.” Draw the following on the board:
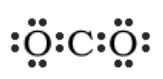
“Looking at this structure, you can see that there are 16 electrons represented, which matches the total from the table. However, carbon is electron deficient. The only way for all three atoms to satisfy the octet rule, is to share MORE electrons and form double bonds.” Change your drawing to look as follows:
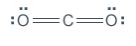
“Just as you did before, change the electron pairs in between oxygen and carbon to lines to represent the bonds. Notice there will be two lines on the left and two lines on the right. These are called ‘double bonds.’ Atoms can form triple bonds as well.” Draw the following:
“All of the examples we have done in this lesson involve atoms that need to share their electrons in order to satisfy the octet rule. When atoms share their electrons, it is called a ‘covalent bond.’”
Example 6
F2 (diatomic fluorine)
|
Atom
|
Valence Electrons
|
|
F
|
7 (2) = 14
|
|
Total electrons
|
14
|
“All molecules are made up of at least two covalently bonded atoms. ‘Diatomic molecules’ are the simplest molecules, made up of two atoms bonded together. ‘Diatomic elements,’ such as fluorine, oxygen, nitrogen, and hydrogen, are found in nature as diatomic molecules composed of two atoms of the element. In this example, the two fluorine atoms each have 7 valence electrons. They bond to share one pair of electrons for a total of 14 valence electrons.”
www.chemprofessor.com/bonding.htm
Part 2
Distribute to students copies of the Covalent Bonding Lewis Dot Structures Worksheets (S-C-4-1_Covalent Lewis Dot Structures.doc) and have students finish them in class. This worksheet can also be assigned as homework and handed in the next day. Refer to the answer key as necessary (S-C-4-1_Covalent Lewis Dot Structures KEY.doc).
Part 3
Display or hand out to students copies of the Bohr model of neon (S-C-4-1_Bohr Model-Valence Electrons.doc). Say, “In 1913, Niels Bohr suggested that electrons travel around the nucleus in specific paths. The paths are located in levels at certain distances from the nucleus. Electrons can jump from one level to another. The Bohr model below shows the electrons in a neon atom. How many valence electrons does neon have in its valence shell?” (8) “Is neon very likely to bond with other atoms? Why or why not?” (No, because its valence shell is full. You can also tell because it is a noble gas on the Periodic table.)
http://www.iq.poquoson.org/6sci/atoms/neonA.gif
Extension:
-
Students who learn best kinesthetically, or need extra practice with the standards, can experience covalent bonding through role playing. You can assign students specific nonmetals and give them each a blank index card. Have them use the periodic table to determine how many valence electrons each atom has, and write it on the index card. Have them move around, looking at one another’s index cards in order to determine which atoms they can bond with.
-
You may choose to discuss electronegativity. Atoms with similar electronegativities will form covalent bonds, either polar or nonpolar. Atoms with very different electronegativities will form ionic bonds, because the electrons are so delocalized.
-
Resonance structures are something that you may discuss if you feel that students need to go beyond the content in this lesson. Some molecules or polyatomic ions have multiple structures, called resonance structures, because they resonate in between them. It is important to note that the molecules do not actually switch from one structure to the next but it is helpful for chemists to write them out as if they do. In actuality, they exist as all three (in the example below) at once. The polyatomic nitrate ion (NO3-) is shown below:
-
