Put the following quote on the board:
-
“Sometimes it seems to me that a bond between two atoms has become so real, so tangible, so friendly, that I can almost see it. Then I awake with a little shock, for a chemical bond is not a real thing. It does not exist. No one has ever seen one. No one ever can. It is a figment of our own imagination.”
-
—C.A. Coulson
-
Charles Alfred Coulson (1910-1974) was a British theoretical chemist who played a central role in the development of quantum theories of chemical bonding.
Say, “Was Coulson right? Do chemical bonds really exist? Can we see them? Can we take pictures of them? Can we see them with a microscope? The answer is yes and no. Bonds do not exist as a line like we have drawn in the lessons nor do electrons exist as tiny back circles, like they we have illustrated in Lewis dot structures. However, connections do exist between atoms, but they are not always linear. Chemical species exist in a 3-D world, but we need a technique to represent them on paper or a computer in a 2-D plane. Hence, scientists have developed models as an illustration technique. It should be noted, however, that in 2009 IBM scientists in Switzerland succeeded in imaging a real molecule called pentacene!” Show students the following image available at the Web site listed.
http://news.cnet.com/8301-13924_3-10319001-64.html
Part 2
Say, “We have discussed one modeling technique used to represent chemical bonding called Lewis dot structures. There are numerous other modeling techniques. We will discuss some of the other most used introductory models, such as ball-and-stick, solid sphere, and computer models.”
Ball-and-Stick Models
Say, “The first step in drawing a ball-and-stick model of a molecule is to draw the Lewis dot structure. Draw the Lewis dot structure for methane (CH4). Is it a covalent molecule or an ionic compound? Both carbon and hydrogen are nonmetals and have similar electronegativities; therefore it is a covalent molecule.” Share with students the following:
|
Atom
|
Valence Electrons
|
|
C
|
4 (1) = 4
|
|
H
|
1(4) = 4
|
|
Total electrons
|
8
|
“This model for methane came from the Lewis dot structure, but it is also considered a structural model because it shows the general structure of the molecule. If we were to draw a ball-and-stick model of methane, the atom’s symbols will be exchanged with shaded or colored balls. Perhaps carbon would be white and hydrogen would be black. In a 3-D model, the geometry of the methane model can be shown. The hydrogen atoms are attached to the carbon atom symmetrically in a tetrahedron formation (pyramidal).” Share with students the following model. Use a modeling kit to construct the 3-D model below.
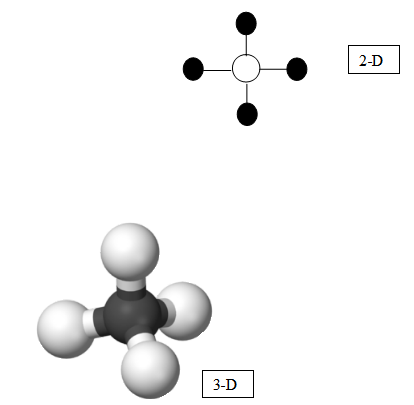
If you have access to modeling kits, use those throughout the remaining examples. You can use or purchase ball-and-stick modeling kits or solid sphere (sometime called space-filling) kits. If you do not have access to these kits, drawing will work. It does not, however, extend to discussions of molecular geometry as well. Gum drops and toothpicks can also work for 3-D models.
Say, “Try the next example: CCl4.” Show students the following:
|
Atom
|
Valence Electrons
|
|
C
|
4 (1) = 4
|
|
Cl
|
7(4) = 28
|
|
Total electrons
|
32
|

http://chemphys.armstrong.edu/P1/a.gif
“This structure is a Lewis dot structure (with no unshared electrons shown) or a structural model. To make a ball-and-stick model, let carbon be represented by a white circle and chlorine be represented by green circles.” Show students the following:
Say, “Let’s try one more example. Draw a ball-and-stick model for carbon monoxide (CO). Carbon monoxide is covalently bonded, with a triple bond between the carbon and oxygen atoms. Let carbon be represented by a white circle and oxygen by a red circle. Even though the Lewis dot structure shows a triple bond, geometry dictates a single line be drawn in the ball-and-stick model.” Share with students the following:
|
Atom
|
Valence Electrons
|
|
C
|
4
|
|
O
|
6
|
|
Total electrons
|
10
|
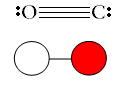
Solid Sphere Models (space-filling models)
Say, “Solid sphere models are a method that is more realistic than ball-and-stick models in that they do have the atoms unrealistically spread apart. Just like the ball-and-stick models, they can be illustrated in a 2-D plane or a 3-D plane. When solid sphere models are represented in a 3-D plane, one or more of the atoms sometimes cannot be seen well.” Share with students examples of a 3-D solid sphere model of methane (CH4).
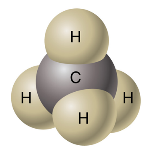
http://wps.prenhall.com/wps/media/objects/439/449969/Media_Portfolio/ch08.html
Say, “Let’s do some examples. You may draw all of the examples in a 2-D plane. Don’t worry about representing the true 3-D molecular shape.” Guide students through the following models of these examples:
Example 1: CO
Lewis Dot Structure:
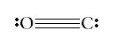
Ball-and-Stick Model:
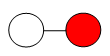
Solid Sphere Model:
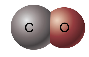
http://wps.prenhall.com/wps/media/objects/439/449969/Media_Portfolio/ch08.html
Example 2: N2
Lewis Dot Structure:
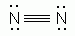
www.sciencegeek.net/Chemistry/taters/graphics/chap6/nitrogen2.gif
Ball-and-Stick Model:
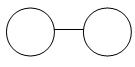
Solid Sphere Model:
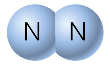
http://wps.prenhall.com/wps/media/objects/439/449969/Media_Portfolio/ch08.html
Computer Models
This lesson does not involve students generating computer-based models, but rather offers pictures of computer based models. Show students copies of the images or display the website listed below each image.
Say, “Some models would be very time consuming and complicated to draw on paper or make with modeling kits. Therefore, many models are made using computer modeling programs. What would a molecule really look like if you could view it through a magical microscope of some kind? A possible answer would be this computer-generated view of hemoglobin, a protein molecule found in red blood cells.” Share the following diagram with students (S-C-4-3_Hemoglobin Molecule-Models.doc).
Solid Sphere Model of Hemoglobin
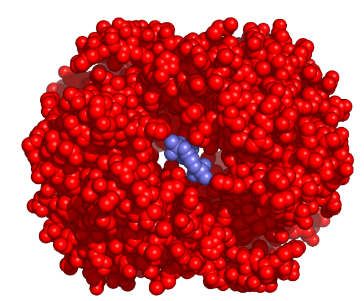
http://nielsolson.us/pictures/23BPG_in_Deoxyhemoglobin.png
“The photo looks like an abstract sculpture, but it does tell us a lot about the shape of the hemoglobin molecule. The ball-and-stick model for hemoglobin is as follows” (S-C-4-3_Hemoglobin Molecule-Models.doc).
Ball-and-Stick Model of Heme Group of Hemoglobin Molecule
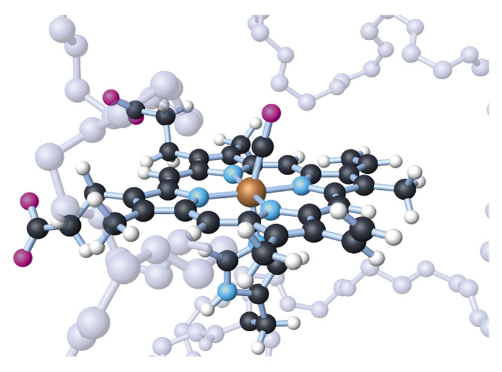
http://cwx.prenhall.com/bookbind/pubbooks/hillchem3/medialib/media_portfolio/text_images/CH25/FG25_07.JPG
Show students an image of a solid sphere model of a DNA molecule (S-C-4-3_DNA Molecule Solid Sphere Model.doc).
Part 3
Distribute to students copies of the Alternative Modeling worksheet (S-C-4-3_Alternative Modeling.doc) and modeling kits, if available. Refer to the answer key as necessary (S-C-4-3_ Alternative Modeling KEY.doc). Have students work individually or in pairs to complete the activity.
Extension:
-
A discussion of forced atomic microscopy is interesting for students who want more information regarding the techniques used to image the pentacene molecule.
-
Scientists want to show a 3-D representation of a 2-D molecule, but on paper. Dotted lines and shaded wedges aid in this task. An example of ascorbic acid is shown below:
http://www.chem1.com/acad/webtext/chembond/cb01.html
-
You can put a copy of the above structure on the board and have students use their modeling kits to construct this molecule, commonly known as vitamin C. Students who finish their modeling worksheet early can make a ball-and-stick model of ascorbic acid:
