Part 1
Tell students, “Forensic scientists often are required to analyze blood spatter at crime scenes. The way in which blood droplets fall on the floor or impact a surface can give the investigator information about the crime. One of the most fundamental blood spatter techniques involves analyzing the size of the blood stain.”
Spread newspaper on the floor. Place three pieces of light-colored card stock on the floor, side-by-side. Using a paper cup, costume blood (S-7-6-1_Fake Blood Recipe.doc), and a disposable pipette, drop “blood” on each piece of card stock at a 90˚ angle (straight down). You should release the first drop from 5 inches above the paper, the second drop from 3 feet above the paper, and the last drop from 6 feet above the paper. You will need to stand on a chair or the lab table to do this. It is fun to do this activity in front of students as a demonstration. It is recommended that you prepare three duplicate cards before students arrive, so that you will have dried blood to reference after your demonstration.
Pass around the dry cards or place them under a document camera, so that students can see the trend. The blood spatters will increase in diameter as the height increases, leveling off at about 7 feet. The picture shown below highlights the trend.
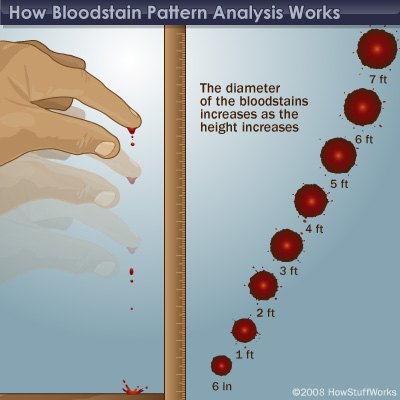
Source: http://science.howstuffworks.com/bloodstain-pattern-analysis1.htm
Variables
Say, “The blood drop experiment is an excellent example of a simple analysis using two variables. Variables are things that change in the experiment. There are two types of scientific variables: independent and dependent. Independent variables can be described as something that the experimenter changes. The dependent variable changes, depending on how the independent variable changes. Let’s determine the variables in the blood spatter experiment.”
Continue by saying, “Which aspect of the experiment did I (the experimenter) decide to change? (The correct answer is the height from which the blood was dropped.) Which aspect of the experiment changed as a result of the experimenter’s change? (The correct answer is the diameter of the blood stain.) Therefore, the independent variable is the height from which the blood fell, and the dependent variable is the diameter of the blood stain.”
Constants
“In order to accurately determine how the dependent variable is affected by the independent variable, the experimenter must keep as many factors as constant as possible. What factors were held constant in the blood spatter experiment?” Have students brainstorm various factors that were or should have been held constant. Acceptable answers may include:
- angle at which the blood was dropped (should have been 90˚)
- amount of blood dropped
- target surface texture
- method of measuring diameter of blood stain
Controls
Tell students, “Generally, there are two types of controls: positive and negative. Controls are a necessary part of a scientific experiment, because they aid in determining the reliability of the results. Positive controls confirm that the procedure is competent, which helps to eliminate false negatives. Negative controls help to regulate false positives. Their purpose is to make sure that the experiment is not displaying a result due to an unrelated effect. Look at the following example experiment and determine the control(s), and at least four constants.”
Say, “For example, suppose that you wanted to measure the effect of an anti-cancer medication on laboratory mice. Twenty identical male mice were separated into two groups of ten. Each group was kept in an identical environment (same amount of light, water, food, noise, and temperature). Each mouse in one group was given 0.5mg of the medication daily, while mice in the other group were given 0.5mg of the food they normally eat.” (Answers: The negative control was the group of mice that was given the placebo [food pill] and the constants were: mouse species, mouse gender, environmental conditions, mouse age, mouse mass, and mass of medication administered.)
Hypothesis
There are several methods to aid in writing a hypothesis. For the purposes of this lesson, you can use the “if/then” technique.
Say, “We are going to write our hypotheses using the ‘if/then’ technique. Start your hypothesis with the word ‘if’.” Using the independent variable first, connect the dependent variable with a statement that contains one of these words or phrases: causes, is related to, is affected by, depends on, etc. “For example, in the blood spatter experiment we did at the beginning of the lesson, the hypothesis could be written like this:
IF you drop blood from a greater height, THEN the resulting stain diameter will increase.
Another example:
The independent variable is amount of water and the dependent variable is plant growth (height). Write an acceptable hypothesis.” (Answer: IF you water plants more, THEN they will grow faster.)
Application
Say, “The goal of this unit is for you to develop, conduct, and interpret an experiment from start to finish. We will all have the same basic parameters to work with, but it is your team’s job to perform the experiment and analyze the results. Working in a team of two, you will make mini ‘rockets’ out of Alka-Seltzer, water, and a film canister. You will study the effect of adding mass, in the form of pennies, on the height the rocket can reach.” (Keep in mind that this unit does not require students to write a formal lab report, but an opportunity to do so is an option in the Extensions section).
The lab will be conducted in Part 2. You can demonstrate the procedure at the end of this part (Part 1), so that students can start the lab at the beginning of the next class session. It is suggested that you do not tell students exactly what to do; rather, give them general parameters and directions as to where the materials are located, which materials they are able to use, and rules you would like them to follow.
Suggested Parameters
- Each team of two may use one empty film canister (go to a local photography store and ask for empty canisters or have students bring them in from home).
- Place 1–2 g of Alka-Seltzer in a dry canister.
- Using clear or masking tape, attach pennies to the bottom of the canister. This will serve as extra weight. Students must start with 2 pennies; they will later extrapolate the height the canister would reach with zero pennies, according to their collected data.
- Add 10 mL of distilled or tap water to the canister.
- Cap the canister immediately.
- Place the canister on the ground, penny side up/lid down.
- Wait for carbon dioxide gas build-up to cause the canister to pop, propelling the base into the air.
- Measure the height the canister reaches.
- Students can decide on factors such as the exact mass of Alka-Seltzer they wish to use, how they will measure the height, how they will attach pennies, and which pennies to use.
- They must do three trials for each penny added (2, 3, 4, 5, and 6 pennies are added). You can have them continue to 7 pennies if you would like them to collect more data. It is suggested that they start with a trial run first, before collecting data.
Hand out the Rocket Lab Worksheet before the start of Part 2 (S-7-6-1_Rocket Lab Worksheet-Student Version.doc).
Have students complete the Variables, Constants, and Controls Worksheet (S-7-6-1_Variables, Constants, and Controls Worksheet-Student Version.doc) for homework.
Part 2
Have students complete Part 1 of the Rocket Lab and worksheet, including data collection, but not calculation of the average height reached by each canister.
Extension:
- This lesson does not require students to write a formal lab report. You may wish to include instructions for writing a lab report, which would include an introduction, materials, procedure, data analysis, and a conclusion. For more on writing lab reports, go to:
http://www.bridgestoliteracy.com/biotech/doc/writinglabreport.pdf
- Students can create launching pads for added control in their experimental set-ups. This would be an excellent engineering component. See Steve Spangler’s version of this experiment at:
http://www.stevespanglerscience.com/experiment/00000068
- Students can alter the temperature of water used.
- Discuss how the choice of pennies affects the mass, due to different densities. If the penny has a date before 1982, it is made of 95% copper. If the date is 1983 or later, it is made of 97.5% zinc.
