Multiple Choice Items:
1. What did J.J. Thomson theorize about the structure of the atom?
|
A
|
Indivisible atoms make up the smallest particles of matter.
|
|
B
|
Orbiting electrons are restricted to specific energy levels.
|
|
C
|
The nucleus occupies a very small portion of the atom.
|
|
D
|
Electrons are like plums in a pudding of positive charge.
|
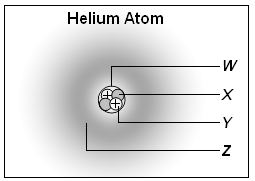
2. Which part of the atom shown below represents the nucleus of the atom?
3. What is the identity of the atom containing 16 protons, 20 neutrons, and 16 electrons?
|
A
|
Oxygen (O)
|
|
B
|
Sulfur (S)
|
|
C
|
Calcium (Ca)
|
|
D
|
Krypton (Kr)
|
4. Which of the following particles, traveling at the same speed, will be bent the most by the magnetic field inside a mass spectrometer?
|
A
|
X+ (mass = 60 amu)
|
|
B
|
R+ (mass = 120 amu)
|
|
C
|
Z2+ (mass = 60 amu)
|
|
D
|
T2+ (mass = 120 amu)
|
5. What is the approximate average atomic mass of the element whose mass spectrum is shown below?
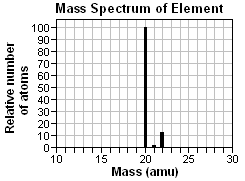
|
A
|
20.0 amu
|
|
B
|
20.2 amu
|
|
C
|
21.0 amu
|
|
D
|
22.9 amu
|
6. Which of the following statements best describes a nuclear fission reaction?
|
A
|
Plutonium nuclei absorb neutrons and split into smaller nuclei with a large amount of energy.
|
|
B
|
Uranium atoms combine with oxygen molecules to produce uranium dioxide and heat energy.
|
|
C
|
Hydrogen nuclei combine together to form larger helium nuclei with a large amount of energy.
|
|
D
|
Cesium atoms spontaneously give off beta particles to produce barium atoms and heat energy.
|
7. The diagram below shows a typical nuclear reaction that occurs during nuclear fission.
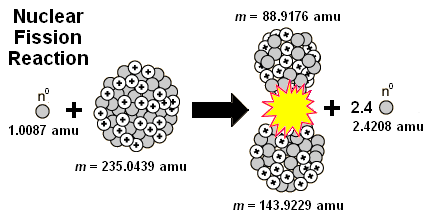
This particular reaction can be described by the formula E = mc² because—
|
A
|
0.7913 amus of mass absorb electromagnetic energy from the surroundings.
|
|
B
|
2.2034 amus of mass turn into electromagnetic energy which is given off to the surroundings.
|
|
C
|
0.7913 amus of mass turn into electromagnetic energy which is given off to the surroundings.
|
|
D
|
2.2034 amus of mass absorb electromagnetic energy from the surroundings.
|
Multiple Choice Answer Key:
|
1. D
|
2. A
|
3. B
|
4. C
|
|
5. B
|
6. A
|
7. C
|
|
Short Answer Items:
8. Ernest Rutherford discovered that a stream of alpha particles (helium nuclei) travels mostly unimpeded through atoms of gold foil. Only a few of the alpha particles are deflected at large angles or bounce backward.
Part A: Explain why the alpha particles did not cause the gold atoms to undergo nuclear fission.
Part B: Explain why so few alpha particles bounced backwards off the gold foil.
9. A student considered using pure uranium in a nuclear reactor cooled by water under very high pressure. The student listed two possible reactions that might occur.
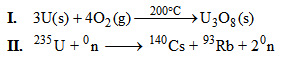
Part A: Identify the type of change that is occurring in each reaction.
Part B: Explain why reaction II gives off much more energy than reaction I.
Short-answer key and Scoring Rubrics:
8. Part A: The alpha particles do not cause the gold atoms to undergo nuclear fission because both alpha particles and gold nuclei have a positive charge and repel each other. As a result, the alpha particle cannot impact the gold nuclei, make them unstable, or cause them to break apart.
Part B: Few alpha particles bounce backward because the gold atoms consist mostly of empty space. The gold nuclei are very small and the probability of an alpha particle hitting one of the nuclei head on is rather low.
|
Points
|
Description
|
|
2
|
- The student’s response clearly and correctly explains why the alpha particles fail to make gold atoms undergo nuclear fission.
AND
- The response clearly and correctly explains why so few alpha particles bounce backward off the gold foil.
|
|
1
|
- The student’s response correctly explains why the alpha particles fail to make gold atoms undergo nuclear fission.
OR
- The response correctly explains why so few alpha particles bounce backward off the gold foil.
OR
- The responses explaining why the alpha particles fail to make gold atoms undergo nuclear fission and why so few alpha particles bounce backward off the gold foil are partially correct.
|
|
0
|
- The student’s response explaining why the alpha particles fail to make gold atoms undergo nuclear fission contains major errors or is omitted.
AND
- The response explaining why so few alpha particles bounce backward off the gold foil contains major errors or is omitted.
|
9. Part A: In reaction I, a chemical change occurs and in reaction II, a nuclear change occurs.
Part B: Reaction II gives off much more energy than reaction I because part of the nuclear mass of the reactant atom is converted into energy (by the fission process) according to the formula E = mc². Reaction I only involves valence electrons being rearranged, which gives off much less energy.
|
Points
|
Description
|
|
2
|
- The student’s response correctly identifies the type of change that occurs for each reaction.
AND
- The response clearly and correctly explains why reaction II gives off much more energy than reaction I.
|
|
1
|
- The student’s response correctly identifies the type of change that occurs for each reaction.
OR
- The response correctly explains why reaction II gives off much more energy than reaction I.
OR
- The response correctly identifies the type of change that occurs for one of the reactions and partially explains why reaction II gives off much more energy than reaction I.
|
|
0
|
- The student’s response identifying the type of change that occurs for each reaction contains major errors or is omitted.
AND
- The response explaining why reaction II gives off much more energy than reaction I contains major errors or is omitted.
|
Performance Assessment:
The following diagram shows a simple mass spectrometer.
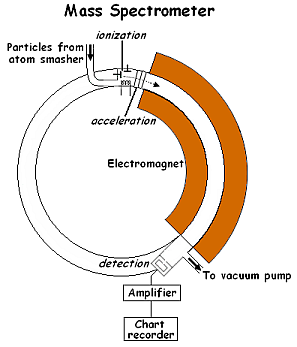
Scientists at the Westinghouse facility in Forest Hills were examining some of the particles entering the mass spectrometer from the atom smasher.
1. Describe how the mass spectrometer helped these scientists make qualitative observations.
2. Describe how it allowed them to make quantitative observations as well.
3. Explain how the mass spectrometer helped the scientists separate and collect different isotopes of an element.
4. Explain why neutrons entering the spectrometer would be absorbed by the shielding around the mass spectrometer rather than being deflected by the electromagnet.
Performance Assessment key:
Answers should be similar to the following:
1. Scientists were able to make qualitative observations using the mass spectrometer by comparing the behavior of the atomic fragments and subatomic particles coming from the atom smasher as they passed though the magnetic field.
–OR–
Mass/charge ratios measured by the mass spectrometer helped the scientists identify the particles and isotopes coming out of the atom smasher.
2. Scientists were able to make quantitative observations using the mass spectrometer by manipulating the magnetic field and using mathematical equations to measure the exact mass and abundance of each particle coming from the atom smasher.
–OR–
The mass spectrometer allowed scientists to determine the mass of different isotopes of an element, count the particles giving an estimate of their abundance, and make possible calculations of the average atomic mass.
3. Since positively charged particles of different isotopes have different masses, the magnetic field can be changed continuously to affect the trajectory of the particles. This allows the particles of different isotopes to be separated and collected in response to changes in the magnetic field.
4. The neutrons would be absorbed by the shielding because they have a neutral charge. The magnetic field can only bend the path of charged particles so neutrons which have no charge are not deflected by the magnetic field and continue to move in a straight path until they are absorbed by the shielding.
Performance Assessment Scoring Rubric:
|
Points
|
Description
|
|
4
|
- The student clearly and correctly describes how the mass spectrometer helped scientists make qualitative observations and quantitative observations.
- The student clearly and correctly explains how the mass spectrometer helped scientists separate isotopes and why neutrons entering the spectrometer would not be deflected by the magnetic field.
|
|
3
|
- The student clearly and correctly addresses three of the four requirements of the question.
- One part of the question contains a major error or is omitted.
|
|
2
|
- The student clearly and correctly addresses two of the four requirements of the question.
- Two parts of the question contain major errors or are omitted.
|
|
1
|
- The student clearly and correctly addresses only one of the four requirements of the question.
- Three parts of the question contain major errors or are omitted.
|
|
0
|
- The student demonstrates a complete lack of understanding or does not attempt to complete the assessment.
- All parts of the question contain major errors or are omitted.
|
