The demonstration requires two test tubes, a test tube rack, a very small amount of MnO2, H2O2, wooden splints, a butane lighter, sand, and fresh liver. Prepare the liver by crushing it in a mortar and pestle with a little sand.
Day 1: Induced Fit Model
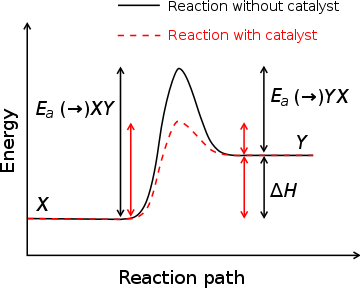
Source: http://upload.wikimedia.org/wikipedia/commons/2/24/Activation_energy.svg
Demonstration: Pour hydrogen peroxide into a test tube until half full. Explain that H2O2 will decompose into water and O2. All it needs is the energy from sunlight, hence the brown bottle to prevent the H2O2 from breaking down.
Define catalyst as “a substance that increases the rate of a chemical reaction by lowering the amount of activation energy needed.” Tell students that you are going to add MnO2, which is a catalyst. In their journals, have students write a prediction of what will happen when you add a small amount of MnO2 to the test tube.
You should see the bubbling on the surface of the MnO2. Set the test tube in a test tube rack and allow the reaction to continue. Ask, “If the catalyst speeds up the reaction, what should be produced?” (oxygen) “What is the test for oxygen gas?” (glowing splint)
Put on your goggles and get the splint ready. Do the test; the flame should relight. “Do you think I’ll be able to do it again, or did I use up all the oxygen?” You will be able to blow out the splint and relight it several times. (O2 is denser than air so it will settle near the surface of the liquid. Be careful not to wet the splint.) Explain that if you needed to, you could filter out the MnO2 and add it to a fresh batch of H2O2. The catalyst would work just as well. Explain that catalysts are chemicals that aid in a chemical reaction by lowering the activation energy.
Show students the graph of the Reaction path, and explain that even reactions that seem to occur spontaneously have to overcome the energy “hurdle.” The beauty of a catalyst is not only does it lower the energy “hurdle” but it isn’t used up in the reaction, so it can be used over and over again. This really speeds up the rate of the reaction. Cells use catalysts, too.
Do the demo again, using potatoes or liver and hydrogen peroxide instead of MnO2. Some students may be surprised that the enzyme still works when the liver (and chicken) is dead. Remind students that the cell is where life begins. The proteins were never “alive”; however, no new proteins will be made in a dead cell.
Instruction
Tell students, “We call biological catalysts ‘enzymes’ and they are made of protein. What is the structure of proteins?” (an amino acid sequence folded and twisted) “Why do you think the twisting and folding is important?” Accept all answers. “Folding is its most important feature because it gives protein a three-dimensional shape.”
Show students how proteins can have multiple twists. Give each pair of students approximately 30 cm of yarn or string. Have them tie the ends to their pen/pencil. Have students hold the string taut and twist the end. Make sure they are not untwisting each other. When the string gets tight from the twisting, have students relax the string a little. The string should twist around itself. Have them hold it taut again. Bring two groups’ string together and let them relax the string together. Remind students that often, proteins are made up of more than one amino acid sequence. Have students compete to see who can get the biggest coil with multiple strings. Using the biggest coil, point out the natural folds, places in the coil that can hold something like a penny or a marble.
Proteins are shaped a similar fashion as the knotted string except the shape of an enzyme is much more specific. The sequence of the amino acids dictates how the protein will twist. The final shape of the enzyme is critical because that is what determines how the enzyme is used by the cell.
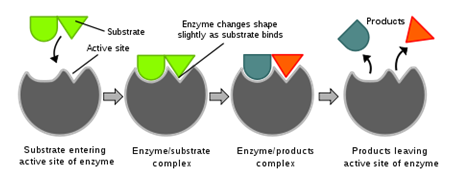
Chemists would say that enzymes provide an active site for the reactants to bind and the reaction to occur. Notice that the active site has a complementary shape for the substrate. When the enzyme binds with the substrate, the enzyme puts stress on its bonds, making it easier for the substrate to break apart, as in hydrolysis. Hydrolysis is a chemical process in which a certain molecule is split into two parts by the addition of a molecule of water... Explain that this is the “Induced Fit” model for enzymes. The induced fit model means that the enzyme will bend or stretch when the substrate binds to it.
Tell the students, “The induced fit model states that when the substrate molecule joins the active site of the enzyme to form an enzyme-substrate complex, the active site slightly changes its shape to accommodate the substrate perfectly.” Have them copy this definition into their notes.
Make two columns on the board. Write “Enzyme” in one column and “Substrate” in the other. Write “amylase” in the first column and “amylose or amylopectin” in the other. Continue adding enzymes and their substrates to the columns (e.g., lactase-lactose, protease-proteins, lipase-lipids). “What is the pattern?” (Enzymes end in –ase. The substrate and the enzyme have the same root.)
Point out that amylase is found in saliva, and amylose and amylopectin make up starch. Lactose intolerance is caused by the inability of the body to produce lactase.
Vitamins, or cofactors, are non-protein compounds that are bound to an enzyme and may help in regulating the enzyme’s activity. Some vitamins are necessary for the enzyme to function. Many of the trace minerals may have the same effect on enzymes.
To close the lesson, ask students to work with a partner to come up with an analogy for the induced fit model, or another way to model it besides the diagram above.
Day 2: Enzymatic Activity
In advance, prepare the 1M HCl and 1M NaOH solution and pour it into dropper bottles. Prepare the pineapple juice, papaya juice, kiwi juice, and apple juice. Filter out pulp with cheese cloth. Prepare meat tenderizer solution (1 Tbsp. meat tenderizer into 1 cup water).
Inquiry Activity
Introduce the scenario by saying to students, “Your parent/guardian (or the person who washes your clothes most often) just bought some new laundry detergent, which claims to have better results removing stains due to enzyme reactions that take place during the wash. You want to help your clothes look good, and find the best settings for the washing machine to promote the enzyme action in the detergent.”
Invite students to brainstorm. “What do we know about enzyme activity?” (It increases the rate of chemical reactions. It is specific to chemicals; one enzyme doesn’t fit all. Has an active site that fits a substrate. Works like a lock and key.)
“What do you need to know, before we proceed with the experiment?” Accept all answers but lead students to ask, “What is making the stains?” Tell students that the most difficult stains are protein. This is important because enzymes are specific and they also need to know the facts:
- Gelatin is made of the protein collagen, which is derived from the skin, white connective tissue, and bones of animals. Gelatin sets to become solid or semisolid.
- Gelatin will not set if the digestive enzyme is present. (You can read this from the box; Jell-O™ warns not to use fresh pineapple, kiwi, papaya, etc., or gelatin does not set.)
- Pineapples contain the enzyme bromelain, which breaks down or digests protein.
“What do you want to find out in this activity?” Lead them to the guiding questions for the activity:
1. Are the clothes washed in hot or cold water? Maybe you want to find the optimal water temperature for the enzymes to work?
2. The water at my house is acidic; it has a low pH. Will the pH affect the enzyme activity of the detergent?
3. The cycles on the washer are timed, so if the stain isn’t digested in 10 minutes, the enzymes will rinse away. Could adding more enzymes to the detergent increase the reaction rate?
4. What if all the clothes in the wash have a stain? Will the enzyme get “used” up after it cleans the stain on one or two shirts?
5. The detergent is expensive. Will another enzyme be as effective?
6. Does an acidic or basic environment have an effect on enzymes?
Introduce the assignment and explain that the purpose of the lab is to study how enzymes are affected by their environment and see if an enzyme can get used up.
Divide students up into groups and hand out the Gelatin Lab sheet to each student (S-B-6-3_Gelatin Lab.doc). Once all members from each group have a lab sheet, assign one of the leading questions above for each group by rolling one number cube. Have them circle the question on their lab sheets.
Extension:
- Students going beyond the standards can Have student pairs write a “Syllable Cinquian” poem about enzymes.
Format:
Line 1— 2 syllables
Line 2— 4 syllables
Line 3 —6 syllables
Line 4— 8 syllables
Line 5— 2 syllables
- Students going beyond the standards can investigate blood clotting in humans. Hand out copies of the Blood Clotting Extension worksheet to students (S-B-6-3_Blood Clotting Extension.doc). Have students make a flow chart showing the cofactors and enzymes involved in blood clotting.
- Students who need more practice should make paper models of the induced fit metaphor of enzyme action (S-B-6-3_Induced Fit Metaphor of Enzyme Action.doc).
