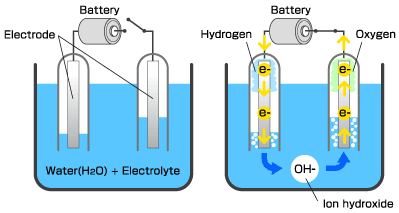
In preparation, set up the electrolysis demonstration as shown. If using a 9-V battery, the battery can also be placed directly in the beaker. Add food coloring to the salt water so students can easily see the gas in the test tube. Completely fill test tubes with salt water, and invert them over the positive and negative terminals or over the wires submerged in the beaker. Wire wrapped around the carbon electrodes produces more bubbles.
Source: http://www.iseof.org/node/4378
Part 1: Basic Chemistry
Tell the class that the components of water can be broken apart with electricity. Show students the electrolysis set-up.
“What is the formula for water?” (H2O)
“Which test tube contains hydrogen and which contains oxygen?” You may need to remind students that the formula means that the ratio of hydrogen to oxygen is 2:1; therefore, the test tube that contains twice as much gas should be the hydrogen. Explain that a chemical formula is basically a “recipe” for a compound. The letters are symbols representing elements from the periodic table. The subscript to the right of the element tells how many atoms are bonded together in each molecule. Explain that the atoms are bonded together, not just mixed together, like when you are mixing the ingredients for a cake. Chemical bonds require energy to form and energy to break. “What are two kinds of chemical bonds?” (Ionic and covalent bonds)
Show students the test for hydrogen by performing the hydrogen “pop test” as follows. Note: Be sure students are at a safe distance from the demonstration. Remove the test tube from the beaker, allowing the water to drain. Since hydrogen is less dense than air, keep the test tube upside-down to contain the hydrogen gas. Light a splint and bring the flame into the test tube. The flame should pop.
Show students that the other test tube contains oxygen. Remove the test tube from the beaker, this time holding the test tube upright and covering the opening with your thumb. Light the splint and blow it out so that it is glowing. Bring the glowing splint into the test tube. The flame should re-light.
Tell students that oxygen and hydrogen are two of the elements found in abundance in living things. Show students the periodic table. Of the 90 naturally occurring elements, organisms are made up of only a few. Show students the list of the elements that are found in the lithosphere. Only eight elements make up 99 percent of the Earth’s crust. Ask, “Do you think that the same elements will be found in organisms with similar frequencies?” Have students brainstorm which elements they think make up living things.
Discuss their predictions and then explain that 99 percent of organisms consist of only four elements.
Show students the complete table. Remind students that that the chemistry of living things is very different from their environments.
|
Percent Composition of Elements
in the Lithosphere and Humans
|
|
Lithosphere
|
Human Beings
|
|
Oxygen
|
47
|
Hydrogen
|
63
|
|
Silicon
|
28
|
Oxygen
|
25.5
|
|
Aluminum
|
7.9
|
Carbon
|
9.5
|
|
Iron
|
4.5
|
Nitrogen
|
1.4
|
|
Calcium
|
3.5
|
Calcium
|
0.31
|
|
Sodium
|
2.5
|
Phosphorus
|
0.22
|
|
Potassium
|
2.5
|
Chlorine
|
0.03
|
|
Magnesium
|
2.2
|
Potassium
|
0.06
|
|
Carbon
|
0.19
|
Magnesium
|
0.01
|
|
All others
|
<0.1
|
All others
|
<0.01
|
The first four elements listed are present in similar numbers for all living things. Plants will have more magnesium and chlorine because those elements are needed to make chloroplasts. The elements that are found in living things in very tiny amounts are called trace elements. Even though they are in minute amounts, the elements perform some specialized task (e.g., vitamins and minerals). For example, iron, a trace mineral, carries oxygen on red blood cells throughout the bodies of some animals. Iron gives blood its red color. Iodine is another trace element; it is used to make thyroid hormones.
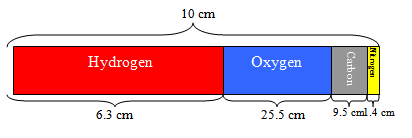
Have students make a pie chart to show the percent composition of the four most common elements found in living things. You might not want your students to measure the angles for a pie chart, and have them make a “bar” chart instead, like the one shown below. This keeps the ratios simple. Alternatively, you could have students make the chart using percent mass. However, some students don’t realize that nitrogen is 15 times more massive than hydrogen and they may assume that nitrogen is present in greater numbers than hydrogen.
Tell students that of the four major elements found in organisms, carbon is the element that makes life possible. They may have heard that we are “carbon-based” life forms from science fiction shows. This is a great description of all organisms.
Tell students, “Carbon is important as a basis for molecules in living things because it can form four bonds with other carbon atoms or other elements. It can also form double bonds and triple bonds. Its molecules can take the shape of long chains, branched chains, network solids, and rings.”
Show students a transparency of Organic Chemistry–Bonding and Structures (S-B-6-2_Organic Chemistry–Bonding and Structures.doc). Carbon’s versatility enables it to form an endless variety of molecules of different sizes and shapes. Carbon forms the backbone of more compounds than any other element. Organic chemistry studies the infinite world of carbon chemistry. At one time, scientists thought that only organisms could make carbon-based molecules, organic compounds, but now we are able to synthesize new carbon-based chemicals in lab. Biochemistry studies only the chemicals used and synthesized by living things. Inorganic molecules and compounds do not contain C–C bonds.
Notice that some of the compounds just have lines, there are no carbons shown. Organic chemists adopted a system where the intersections of the lines represent carbon atoms. They also don’t write each hydrogen atom in the molecule. Chemists know that the carbons will have as many hydrogen atoms as needed to make four bonds.
Students may need a brief review of bonding. Use the periodic table to show how the group number of the representative elements relates to valence electrons. Have students draw Lewis dot structures for the first and second periods, and show that the only the unpaired electrons can form bonds. Remind them that the noble gases do not form bonds.
Ask students, “Do you recognize a pattern in the number of bonds that can form for each group?” (1 bond, 2 bonds, 3, 4, 3, 2, 1, 0 bonds)
Ask, “Which group can form the most bonds?” (Group 14)
Have students think-pair-share about the most important aspect of carbon in biochemistry and then summarize their discussion about carbon in their science journal.
Part 2: Polymerization and Macromolecules
In preparation, bring in a variety of carbohydrates, including bread, potatoes, dry pasta, fruit, vegetables, honey, table sugar, soda, milk, wood bark, mushrooms, and an exoskeleton from a shrimp or insect. Bring pictures if it is not possible to get the actual items. Make double-sided copies of the Foldable―Macromolecules PDF (S-B-6-2_Foldable-Macromolecules.pdf).
Ask, “What elements make up humans and how do we replenish them?” (Oxygen, hydrogen, carbon, nitrogen make up 99% of living things. We get these elements from food and the air we breathe.)
Have all the items laid out for students to see or show them pictures of the carbohydrates. Ask, “What do these items have in common?” (Answers will vary, but food, starches, or carbohydrates will be common.) Tell students that they are all carbohydrates, or kinds of sugars. Carbohydrate means carbon and water, which are the components of all sugars.
Optional Demonstration: Show students that “carbohydrate” describes sugar well. Under a chemical hood, put a small amount of table sugar in a beaker and add a small amount of concentrated sulfuric acid, H2SO4. CAUTION: Concentrated H2SO4 is extremely caustic. Stir the contents until the sugar turns black. When steam is generated, remove your hand and close the hood. A carbon tube emerges as most of the water is boiled off. Explain that sulfuric acid is a strong dehydrating agent pulling the water, H2O, out of the sugar, C12H22O11, leaving only the carbon. Note: There will be some residue left in the beaker, so it is best to use a reused jar or old beaker that can be thrown away after the demonstration.
Tell students, “Carbohydrates, like all macromolecules, are polymers. A polymer is a large molecule made up of smaller molecules.” Demonstrate this with a paper clip. Remind students that molecules are made up of more than one atom. Link two paper clips together and show that the molecule is growing and since the monomer (single molecule subunit) usually contains carbon, the chains can be very long. Demonstrate this by showing the paper clip chain. Point out the chain is made up of identical monomers that are linked together (A-A-A…). Sometimes polymers consist of subunits arranged in a repeating pattern (A-B-A-B…). Polymers may form a single chain or have many “pendants” hanging from it. Add paper clips that hang down from every other clip in the chain. Polymers can be huge molecules with thousands of monomers making them up.
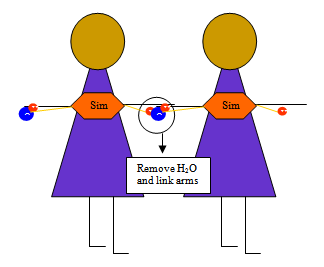
Have students model the process by wearing a hexagon shape labeled “Simple Sugar" with an H and an OH attached one on each arm (see drawing above). Since there is only one simple sugar, it is a monosaccharide. Mono is Greek for one, and saccharide is Greek for sugar. Have another student come up. Remove the H+ from the first student and the OH− from the second, forming a water molecule. Tell students that this is called condensation or dehydration synthesis because water is removed to build or synthesize a molecule.
“How many sugars are linked together?” (two) Guide students into calling the new molecule a disaccharide. Di- is Greek for two. Continue adding students, asking comprehension questions each time you add another student. “What molecule has to be removed?” (water) “What is the process called when water is removed to put two molecules together?” (dehydration synthesis) “What is another word for the simple sugar?” (monomer) Have students demonstrate the process.
“Predict what would need to happen to break the polysaccharide apart.” (add water) Explain that breaking a polymer apart into simpler molecules is called hydrolysis, where hydro means water, and -lysis means to break apart. Have students demonstrate this process and then move back to their seats.
Give students the blank Foldable―Macromolecules handout to complete. Have students complete the rest on their own. They need to include the elements that make up the molecules, how the macromolecule is used by organisms, examples of the molecule, description or drawing of the monomer, and how the polymer is made. Note: The answers provided on the Foldable―Macromolecules resource are intended as a comprehensive key. Students should be expected to provide varying degrees of information on this activity.
When the foldables are complete, ask students questions about the information gathered during the activity: “Which macromolecule contains mostly hydrogen and carbon?” (lipids) “What is used for energy storage?” (carbohydrates and lipids) “Why are nucleic acids important?” (genetic code, regulate cellular activities) “What element do carbohydrates contain that lipids do not contain?” (oxygen)
This is also a good time to discuss nutrition. Ask, “What macromolecules can be used for energy?” (carbohydrates, lipids; nucleotides if students were shown ATP) Explain that before a cell can use anything for energy, it must be converted into a simple sugar first. Proteins, too, can be converted, but usually only in emergencies. “Are no-fat diets healthy?” (No, you need fat to make hormones.) “Experts say that to burn body fat, you need to do aerobic exercise (exercise that makes you breath hard and increases the heart rate). Why does this makes sense biologically?” (Lipids need the oxygen inhaled in order to be converted into carbohydrates the body will use.) “If an animal does not eat enough food, their bodies begin to use stored fat for energy. What happens when their bodies run out of stored fat?” (Their bodies will use protein as energy, such as muscles.)
Part 3: Chemical Testing for Macromolecules
In preparation, blend all the ingredients of the kid’s meal until it forms a liquid suspension. Add water if necessary. Both milk and thesoda will test positive for simple sugars (because Benedict’s solution reacts with lactose) but the coloring of some colas may make it difficult to read the results. Divide the contents into enough flasks so each lab station receives one. Place stoppers in the flasks. Label each flask, “kid’s meal.”
To prepare the starch solution, dissolve 20 mL of corn starch in 100 mL of cold water. When starch is dissolved, heat the mixture until it is smooth and transparent. When cool, add to flasks for each lab station. Label each flask, “starch”. To prepare protein solution, dissolve 0.5 g of plain gelatin in 100 mL of very hot water. Stir mixture until dissolved. When cool, add to labeled “protein” flasks for each station. Alternatively, add 1 egg white to cold water. Mix well. To prepare the glucose solution, dissolve 2 grams of glucose into 100 mL of warm water. Stir mixture and pour into labeled “glucose” flasks.
You may choose to have only one or two containers of each mixture and have students pour solutions into the test tubes from one central location, but you will have lines of students waiting for materials. Have enough test tube brushes and soap for each station and encourage students to clean as they go.
Prepare the reagents if necessary and pour into enough labeled dropper bottles for each lab station. Lugol’s Iodine solution should be placed in an amber dropper bottle.
Finally, begin boiling the water for the water bath. It might be more efficient to have two water baths available to reduce students’ wait time. Add more water to the water bath beakers periodically and between classes.
McMush Lab
Explain that one complaint that doctors have about astronauts is that they don’t eat enough in space because they would rather work than eat. Their bones are already comprised from microgravity; add to that lack of calcium and Vitamin D, astronauts become weaker. This can’t happen on the long journey to Mars. One idea is to use liquid food, packed with nutrients, so astronauts don’t have to take too many breaks to eat. Note: Show students the McMush Mixture. Tell students that this cosmic milkshake was made by blending together the ingredients of a kid’s meal. Scientists will work on the taste later; right now we are focusing on the nutrients.
Divide students into their lab groups and give them copies of the lab (S-B-6-2_McMush Lab and KEY.doc). CAUTION: Caution students about the reagents that they will be using, requiring the use of a lab apron and goggles. Monitor students throughout the lab, answering questions about procedures and test results.
When the lab is complete, allow students time to clean up their lab station and discuss the questions with their group. Collect the lab handouts before they leave.
Extension:
- Students going beyond the standards can investigate three trace elements found in humans, explain how they are used by the cells/ body, and what can happen if individuals do not get enough trace elements in their diets. Have students present their findings as a “Wanted…” poster.
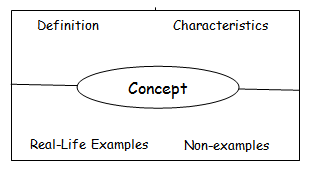
- Students who need an opportunity for additional learning may benefit from the Frayer Model vocabulary cards for the four types of macromolecules. After making the cards, have students study or play games with them such as Password or Pictionary.
Frayer Model vocabulary cards: